領先於世界將「未來」商品化並推向新次元
2011年,由華盛頓大學醫學部(密蘇里州,聖路易斯)發生生物學部門·醫學部門教授今井真一郎為中心的團隊,報告了使醫療業界大幅發展的NMN,(簡稱NMN、β nicotinamide mononucleotide、β NMN、煙酰胺單核苷酸:一種維生素B3,也包含在自然生物中,輔酶NAD:煙酰胺腺嘌呤二核苷酸,nicotinamide adenine dinucleotid的前體,以下稱為NMN)該物質的存在及其令人驚訝的效果。
NMN包含在維生素B族中的維生素B3中,存在於所有生物細胞中。現在,關於NMN的研究在世界範圍內持續著,受到了非常多的關注。 NMN本來是體內自然生成的物質,據說是隨著年齡的增長,其在體內的生成能力逐漸減少。
MIRAILAB生物科學的NMN是世界首個「學術界品質」的NMN
本公司的NMN,實施了世界首次人類安全性試驗,也是在國立大學醫學部長期(24週)的臨床研究中確認了Sirtuin遺傳基因的發現,褪黑素的增加,和其他生長激素類的增加的NMN。
其業績和純度等得到了很高的評價,捐獻提供的NMN製品,被華盛頓大學醫學院採納為由美國國防部(DOD)資金提供的新臨床研究的試驗食品,是NMN配合產品中的世界最高峰,也是「學術界品質」的NMN。
世界最高99%以上的純度
本公司的NMN已被各研究機構確認純度為99%以上,也確認了對人類和囓齒動物均具有高度的安全性。此外將含高純度NMN保健食品商業化的,世界上就還有另一家公司。
NMN品質所指的高「純度」,需要注意少量的「雜質(混合物)」的理由,和關於「雜質物質」的本性,請關注以下內容。
●NMN配合產品中標記含有的NMN「純度」表示正確嗎? NMN中含有的「雜質物質」究竟是什麼?
在對NMN的關注越來越高的今天,除本公司以外,全世界也有許多NMN混合產品氾濫。但即使是被稱為NMN配合的產品,也需要充分注意因各產品所使用的NMN的品質有不同之處這一事實。
●因低純度而引起的悲劇
含低純度NMN的產品中必然會含那麼多的「雜質物質」。目前還不清楚在使用這些含雜質物質產品時會產生什麼樣的負面影響。
舉個極端的例子,有10,000人以上健康受損,並有100人以上被奪去生命的砷牛奶中毒事件(1955年)的原因是,作為安定劑使用的添加劑中含有5%左右的雜質物質。
根據這些過去的教訓的現代,美國食品藥品管理局(FDA)也提出了「為了食品的安全性,有必要極力排除雜質物質」的建議。
●關於NMN純度標記 ~絕對性測量方法和相對性測量方法~
關於NMN的純度,有些產品標記為99.9%等。乍一看,給人一種純度非常高(含雜質物質少)的印象。但是,這個99.9%的數值表示什麼,是否有明確的說明呢?
●關於絕對性方法測量的純度
作為可靠性的高純度的試驗方法,可以檢測出「在原料中檢測到的所有物質中,NMN的比例是百分之幾」。根據這種絕對性的測定方法進行純度標記,是本公司對純度標記的定義。
●關於相對性方法測量的純度
一般情況下,當測試機構進行純度檢查時,「作為標準的NMN原料」的純度為100%時,這只是一個相對值。令人驚奇的是,「作為標準的NMN原料」,製造商是可以自己提供,其純度是尚未公開的。
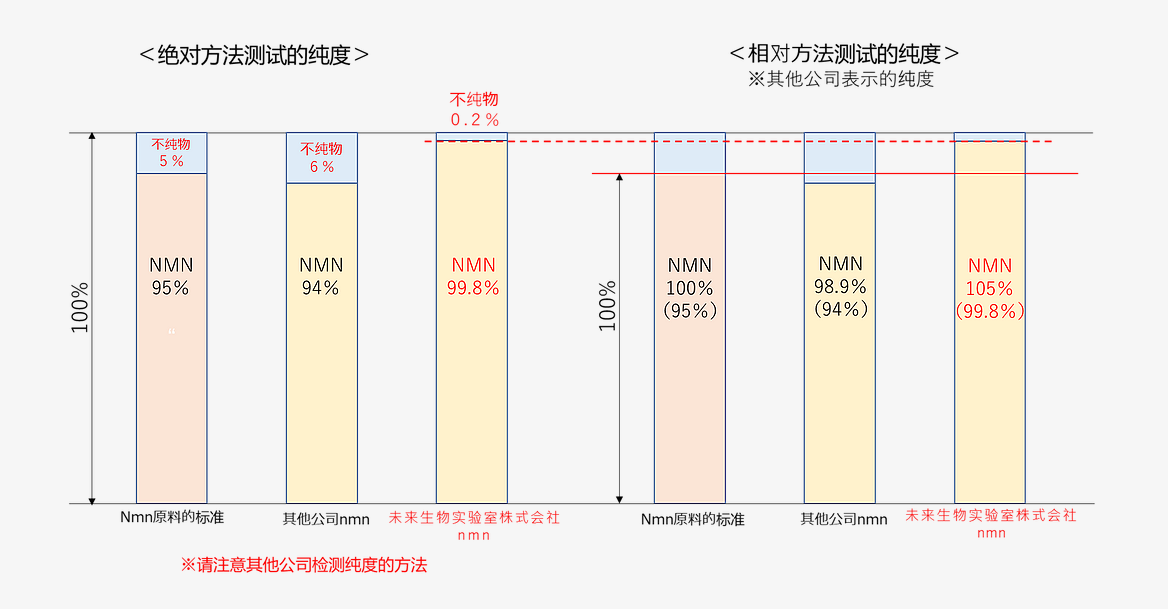
如上圖所示,如果使用絕對性方法測量的「標準NMN原料」的純度為95%,其他公司的NMN的純度為94%,則使用相對性測量方法的純度為98.9%。
另一方面,本公司的NMN純度為99.8%,如使用與上述相同的相對性測量方法進行檢查時,純度為105.0%。
在相對性方法測量中,受「作為標準的NMN原料」本身的品質所左右,因此,乍一看即使是印象良好的檢查結果數值,也不能斷言其含雜質物質少。
希望所有的企業都能用這種公平的方法進行純度標記,但現狀是各家公司沒有明確說明詳細內容情況下的標記。
●根據使用絕對性測量方法去比較分析NMN純度
一般社団法人Productive aging研究機構(IRPA),對4家使用NMN公司的NMN原料純度
進行了絕對性方法測量。
這些測試結果,表明了本公司的NMN具有世界上最高的純度和品質,可以安心使用。
一般社団法人Productive aging研究機構(以下為IRPA),4家公司的NMN純度進行了LC-UV(※1)NMR(※2)2種方式測試,絕對性方法的測量各種結果顯示只有本公司的NMN是99%以上。
結果表明,其他三個公司的NMN包含很多雜質
※1 LC-UV(高效液相色譜-紫外線吸收光譜法)
※2 NMR(核磁共振波譜)
NMN 樣品的LC-UV Analysis測試、NMR Analysis測試結果請參考下圖對照表。
●NMN中含的雜質物質分析
一般社団法人Productive aging研究機構(IRPA)對4家使用NMN公司的NMN原料中的雜誌物質
進行了測量分析試驗。
與進行純度分析時相同,首先用LC―UV方法進行分析,此時,通過用可以檢測出雜質分離峰的質量分析法(MS)試驗,同定雜質成分的方法進行調查,其結果,在本公司的NMN原料中,僅檢出了含少量「煙酰胺」的雜質物質。
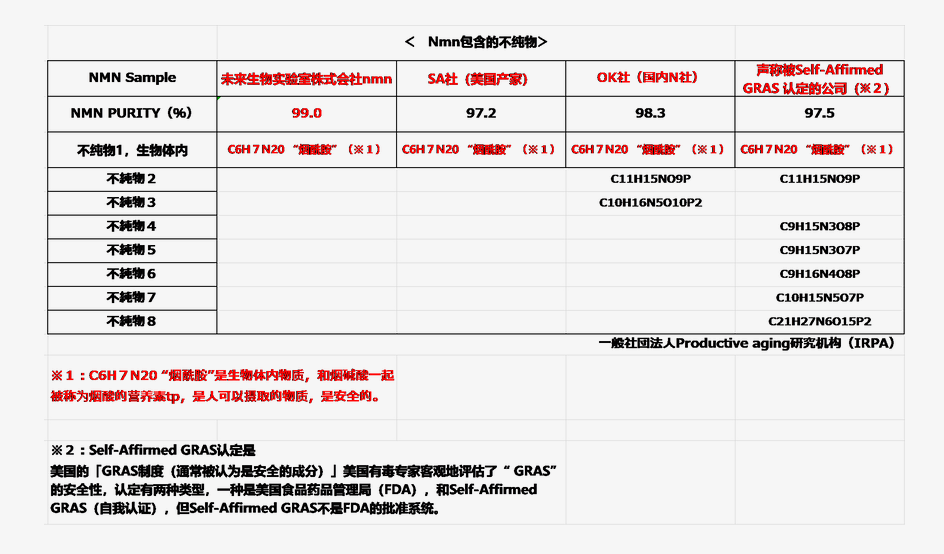
4家公司的NMN中含全有作為雜質的「煙酰胺」,是生物體內與菸酸一起被稱為菸酸的營養素物質,人類可以攝取的安全性物質。
另一方面,「OK社」和「Self-Affirmed GRAS獲得認證的公司」的NMN中,作為雜質物質的「煙酰胺」以外,還含有其他多種物質。
特別是,「Self-Affirmed GRAS獲得認證的公司」的NMN中,「煙酰胺」之外還含有6種雜質物質,
其中有在生體內不可想像的物質存在。長期攝入這些含有大量雜質的NMN的產品可能會對身體產生影響。
特別是,「Self-Affirmed GRAS獲得認證的公司」的NMN中,「煙酰胺」之外還含有6種雜質物質,
其中有在生體內不可想像的物質存在。長期攝入這些含有大量雜質的NMN的產品可能會對身體產生影響。
請務必注意,即使是標記為含高純度NMN,但實際上其含有何雜質物質,另外還有含純度低且安全性尚未得到確認的NMN的產品也在市場上銷售。
◆一般社団法人Productive aging研究機構(IRPA)◆
https://www.irpa.ne.jp/
關於MIRAILAB生物科學進行的安全性、評價及其他各種試驗的實施
■廣島大學大學院生物醫學與健康科學研究院:2019年3月31日結束
煙酰胺單核苷酸(NMN)的長期攝取對人體產生的影響
—安全性方面沒有問題
https://upload.umin.ac.jp/cgi-open-bin/icdr/ctr_view.cgi?recptno=R000029616
■日本食品分析中心:2018年7月30日結束
・急性口服毒性試驗—無異常或死亡案例(小鼠)
・反向突變試驗—陰性(無)
・皮膚一次性刺激實驗—沒有問題
・眼球刺激性實驗—無刺激
■DRC株式會社:2015年11月23日結束
・含有白藜蘆醇和煙酰胺單核苷酸的食品
通過攝取組成物,對脂質改善效果的實驗:
(對人的臨床研究)—安全性方面沒有問題
■日本食品分析中心:2015年8月17日結束
・急性 服毒性試驗—無異常或死亡案例(小鼠)
・反向突變試驗—陰性(無)
・皮膚一次性刺激實驗—沒有問題
・眼球刺激性實驗—無刺激




